Product quality and safety
People around the world trust us to produce and market safe and effective medical products. We deploy rigorous processes, procedures and patient information throughout the product life cycle, beginning with clinical trials, by conducting product safety assessments, effective risk management, and transparent risk communication. This helps ensure our products serve a diverse patient population, meet our quality standards, are used compliantly and safely, and are protected from illicit actions such as counterfeiting.
Clinical trials

Our commitment to product safety is built into our products from the very beginning through our clinical trials.
As a sponsor of clinical trials, we are accountable for submitting preclinical and clinical data to regulatory agencies in support of our marketing applications and ensuring the safety and well-being of trial subjects in any interventional study. We review all safety information regularly and any serious adverse events on an expedited timeline. We also use expert panels to adjudicate individual cases for products with specific known risks.
Organon complies with Good Clinical Practices (GCP) in the United States and with the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use and Guideline for Good Clinical Practice (ICH GCP). ICH GCP are internationally accepted principles and practices in the design and conduct of clinical studies. In the United States, the standards are known as GCPs. In addition, Organon complies with the current Declaration of Helsinki, which are standards that sponsors of clinical trials comply with in order to protect the safety and well-being of participants in clinical trials.
Diversity in clinical trials
In line with our commitment to women, we understand the importance of conducting clinical trials with diverse subjects, ensuring medicines are well understood and developed in target populations as well as subpopulations that may respond to treatment in different ways.
We develop plans to inform recruitment strategies to comply with U.S. FDA regulations governing diversity in clinical trials. For Organon, the plan is to have diversity action plans in place for phase 3 clinical trials as required. Our Clinical Development Leadership Team is responsible for ensuring that diversity in clinical trials is integrated into Organon’s trial planning process. The leadership team regularly reviews ongoing and planned studies.
Our ongoing efforts to embed diversity and inclusion strategies into clinical development cover a range of key areas. We develop all clinical trial plans to inform recruitment strategies that, at minimum, comply with U.S. FDA regulations governing racial and ethnic diversity in clinical trials. Wherever allowed, we also seek to balance gender representation and engage participants across a variety of age groups.
At the clinical program level:
We seek to understand patient populations, with a particular focus on epidemiology of diseases and underserved populations that feel their impact. If applicable, we involve patient advocacy groups in our planning process.
As we design studies:
We undertake an evidence-based review of protocols to identify any potential barriers to participation by diverse groups. We also characterize and validate the makeup of intended patient populations based on analyses of real-world healthcare and demographic data. Finally, we assess our protocols against known barriers to identify any potential issues as part of our data-informed protocol assessment.
As we select sites:
We factor in the knowledge and experience sites have with diverse populations alongside other considerations outlined in our diversity, equity, inclusion, and belonging (DEI&B) goals, including expanding our presence in developing countries. When we activate a site, we communicate intended enrollment and the demographics of the disease to the research organizations running the trials. We also include diversity training at our investigator meetings and on-site visits.
We support recruitment and retention efforts through community outreach and efforts to understand the patient journey via continuous engagement and monitoring. We work to ensure our recruitment strategy, tactics and materials are informed by the motivations, concerns and preferences of the populations we wish to reach. Our Electronic Case Report Form includes questions that help us track potential concerns and enhance patient engagement throughout the life of the trial, wherever in the world our trials may be conducted.
Learn more about our work in innovation for women’s health and access to medicines and healthcare
Product quality
People trust us to produce and market safe and effective medicines and medical devices. To preserve this trust, our products undergo rigorous quality checks before release and continue to be closely monitored once they are on the market. We consider product quality one of our top priorities, and we maintain policies and structures that help ensure we meet this expectation.
We are focused on consistently improving our product quality systems and processes applicable to our product portfolio, in addition to responding to the complex and evolving regulatory and compliance framework applicable globally.
This commitment begins with our robust quality management systems (QMS), which are designed to promote and facilitate regulatory compliance and operational excellence throughout a product’s life cycle, from upstream product inputs to downstream finished products, including manufacturing and packaging. We seek to meet and exceed governmental requirements through our internal product safety standards. We expect our contractors, suppliers and other partners to adhere to our product quality standards and regulatory requirements related to quality, and employees are empowered to help ensure these partners share our rigorous approach.

Designated employees participate in the training required to adhere to current Good Manufacturing Processes (cGMP). We also have policies and procedures in place to identify deviations from cGMP, which we report promptly in accordance with regulations and our product quality standards.
Global product quality oversight
We also help to ensure that there is accountability for our quality initiatives by implementing executive oversight. The senior executive for Manufacturing and Supply oversees the quality organization for commercial products, working closely with the Global Head of Quality. Our quality group manages quality operations at our six internal facilities and oversees quality operations at our contract manufacturing sites and in our distribution networks.
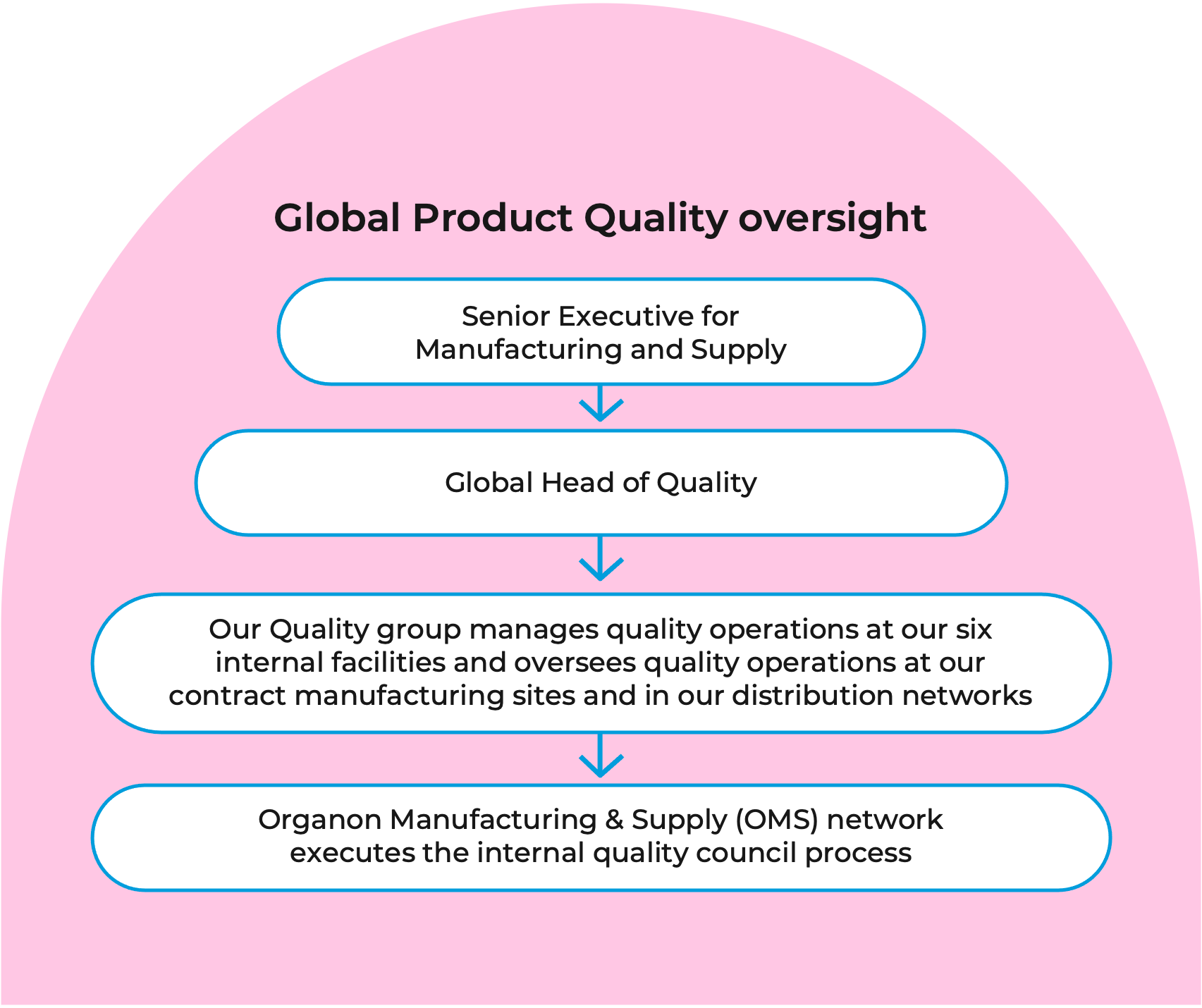
These functions represent the end-to-end quality oversight of the manufacture of our product portfolio. We also monitor key metrics related to maintaining and sustaining the quality systems and culture on a monthly basis. This is part of the internal quality council process that is executed through the Organon Manufacturing & Supply (OMS) network.
Our products are tested throughout the manufacturing process prior to their approval and release to the market. This testing is governed by a regulatory framework as well as approved specifications and test methods in alignment with our product regulatory submissions. Organon maintains a supplier and contractor quality management program embedded within our QMS that defines the oversight of suppliers, service providers and contract manufacturers that impact the quality of the finished product. We investigate product quality complaints and take measures to address any issues, including market actions such as recalls, when appropriate.
Learn more about our performance in our ESG reporting center
Product safety management and oversight



Developing products
We seek to ensure that our products are used safely worldwide by conducting product safety assessments, effective risk management, and transparent risk communication.
Any suspected changes to the safety profile of any product are evaluated by a cross-functional team of experts and escalated to senior management as appropriate. Should a change in a product’s safety profile be identified, we are committed to working expeditiously to implement remedial measures, including escalation to regulatory agencies, updates to the product label, further investigation, implementation of risk-minimization measures, or, in rare instances, product recalls as appropriate.
All Organon employees, contractors, and vendors are required to complete annual training on reporting adverse events in support of our safety profile evaluations.
Organon’s Product Integrity Program Office (program office), within Security & Resilience, manages all reported counterfeit, diversion, tampering, and intellectual property events for the Company. The program office actively identifies, assesses, and responds to all indications and reports of suspicious events. Our goal is to quickly detect, assess and disrupt events that impact patient safety and help protect our company’s reputation from the negative effects of counterfeit and illicit medicines.
Our product integrity strategy proactively seeks to protect the safety and security of our patients through activities throughout our supply chains.
We have invested in serialization in certain markets — adding a barcode with a unique identification number on each package going to market — to secure our supply chain and prevent or detect counterfeiting and diversion. When serialization is complete, stakeholders across the supply chain will be able to scan the code and verify it as a serial number corresponding to an Organon product. This investment will continue over the next few years as we go live in more markets.
The Global Security & Resilience team offers regular employee training on the counterfeit, diversion, and tampering reporting process. Our Security & Resilience team is also partnering with internal and external stakeholders — including packaging engineers, artists, and other third parties — to develop secure packaging designs for our products, including tamper evidence options and other anti-counterfeiting features.
The final design for a product depends on its external risks according to market, region, location, country regulations, and therapeutic area. Our labeling strategy also requires high-quality and compliant labeling documents, which help ensure the safe, effective and informed use of products for patients and healthcare providers globally.
Our product integrity program focuses, in part, on raising public and stakeholder awareness of the risks of counterfeit medicines. We partner with law enforcement agencies, the national health authorities, other pharmaceutical companies and organizations focused on security, patient safety and public health to advocate for high-priority anti-counterfeiting policies. As part of our awareness program, internal stakeholders were trained on patient safety dangers related to counterfeit and diverted medications. We deployed the “Product Integrity – How to report Counterfeit, Diversion, and Tampering issues” course in our learning management system (LMS). The course represents an extensive opportunity to create awareness of the threats of illicit medicines among all our founders, as well as how to report such events.
Organon uses proportionate efforts, where suspected and feasible, to analyze and detect counterfeits in regions around the world, including the use of forensic detection devices in the field. In addition, we conduct forensic analysis of questionable products to determine whether they are counterfeit, diverted or otherwise illicit, which allows us to gather intelligence on counterfeiting operations and understand the threats to public health.
As counterfeiters improve their skills and techniques, our forensic scientists have pioneered the use of several analytical tools for the detection and characterization of counterfeit medicines and continue to explore new analytical techniques to increase their forensic testing capabilities. We cooperate with relevant government agencies, other pharmaceutical manufacturers, wholesalers, distributors, health professionals, consumer groups and key related organizations in the campaign against counterfeit medicinal products. We also educate the public about the risks of counterfeit products and how to protect against them.
Across the industry, product integrity events vary based on the size of a company’s portfolio and where the products are distributed. Counterfeit, diversion, supply chain security, tampering and brand security events have led to law enforcement partnerships, arrests and seizures of counterfeit or illicit Organon products. Learn more about our performance in our ESG reporting center.
Our product packaging labels contain information on possible side effects and, if appropriate, how to avoid some potential health problems.
Our product packaging and our corporate website contain contact information for patients, caregivers and health professionals to report adverse experiences in the United States. Outside the United States, adverse experiences are reported in accordance with any additional local country laws and practices.
Our Global Pharmacovigilance and Safety Science (GPV) group leads the Risk Management & Safety Teams (RMST) for all products inclusive of biologics, medicinal products and medical devices in development or on the market, throughout the product life cycle. GPV is responsible for the development of proactive clinical safety risk-management strategies, including the Risk Management Plan, which is a regulatory requirement in many countries for marketed drugs and devices.
When urgent changes to our labeling are required to protect patient safety, we work with regulatory authorities to communicate to healthcare professionals in a timely manner so that they can inform patients through appropriate mechanisms. Communications to healthcare professionals may include “Dear Healthcare Provider” letters and media statements.
Our labeling personnel receive training on appropriate policies and procedures at onboarding and every time our labeling systems are updated (typically, every quarter).
The ongoing oversight and monitoring of our product labels is a major focus of our safety efforts.
Our label review teams monitor information on our products and work with our product Risk Management and Safety Teams to develop or update product labeling. We regularly communicate relevant information to regulatory authorities worldwide.