Business ethics and compliance
It takes all of us at Organon working together to create an ethical culture and ensure that we act with integrity in everything we do.
Our Code of Conduct
Our Code of Conduct outlines our ethical expectations and commitment to integrity. The Code of Conduct helps us earn and ultimately keep the trust of our customers, investors and partners, as well as the communities where we operate around the world.
It represents the very core of our character as a company.
The Office of Ethics and Compliance actively supports our commitment to the highest standards by facilitating behavior consistent with the Code of Conduct and by fostering a culture that promotes the prevention, monitoring, detection and resolution of potential misconduct. Our compliance professionals support our sales and marketing efforts worldwide.
Our values are defined by robust global policies. All employees and contractors undergo mandatory annual training on our Code of Conduct and receive training on business practices and compliance, according to their roles and responsibilities. Our Board is also trained on the Code of Conduct on an annual basis. Our Code of Conduct requires that our employees and contractors follow not only the letter but the spirit of the laws, industry codes and policies in the countries where we do business and seek help when needed.
Our commitment to ethical practices and compliance is demonstrated through our strict adherence to federal and state transparency laws when reporting payments and transfers of value to U.S. healthcare professionals and U.S. healthcare organizations. We maintain full transparency via the accurate and timely reporting of all relevant information, which the public can access through the U.S. government’s open payments site. Our ethical practices and compliance are further demonstrated by disclosing our transfers of value to healthcare professionals and healthcare organizations in those countries where we are obliged or have undertaken to do so.
Our policies also require that our employees and contractors are honest and accurate in what they say about our company and our products and services as well as fair and transparent in their business dealings. They are empowered with the tools they need to identify and report potential issues without fear of retaliation.
Ethics reporting
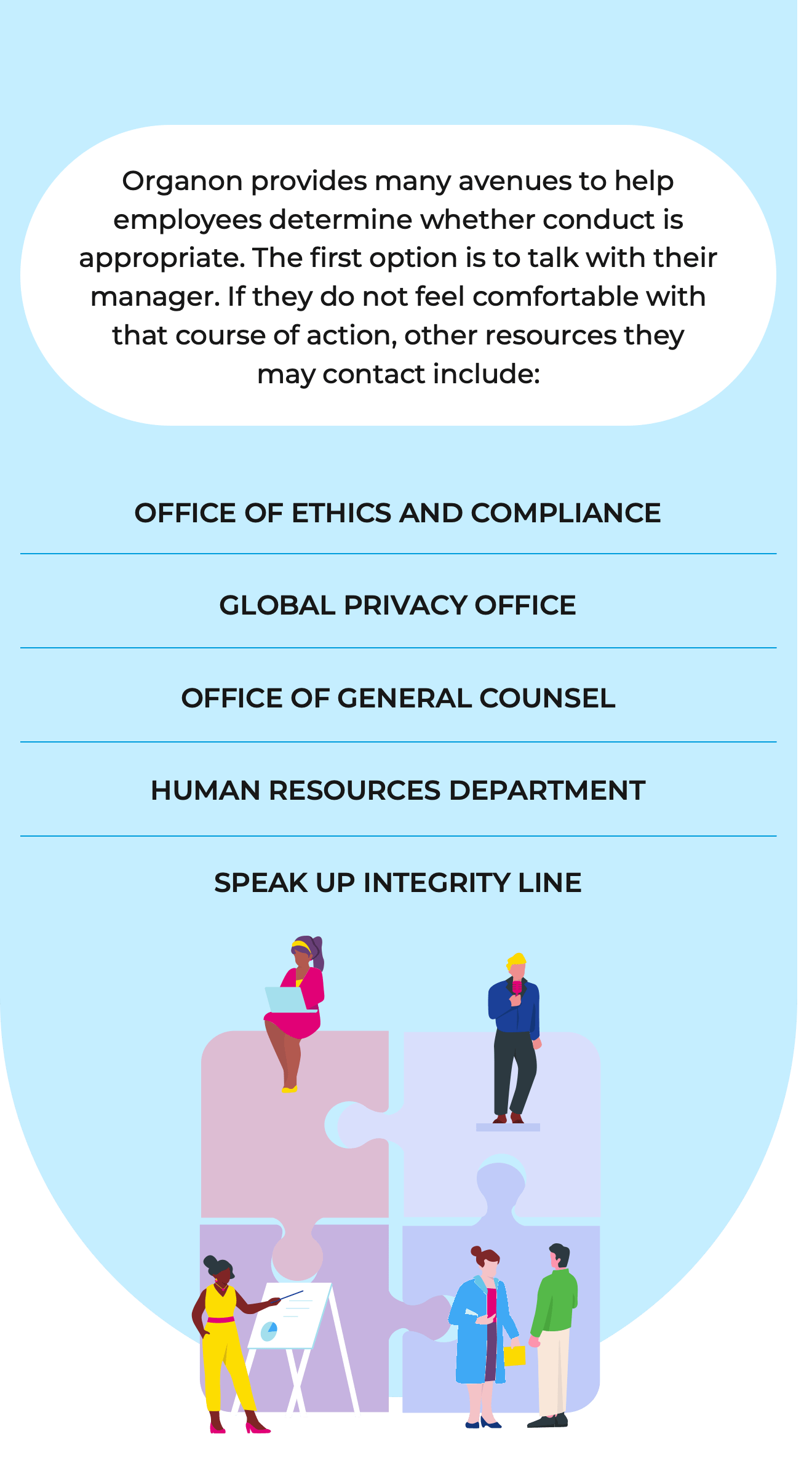
We encourage our employees to speak up about potential violations of our Code of Conduct, policies, procedures, and the law or other misconduct. We provide a safe environment in which our employees are encouraged, prepared and empowered to raise concerns.
Speak up

Employees and contractors anywhere in the world who are concerned about an ethics issue can use the Speak Up ethics hotline, which is operated by a third-party service provider. Speak Up lets employees address work-related concerns online, via a mobile site or over the phone 24 hours a day, seven days a week, in 29 different languages. Reporting employees can choose to remain anonymous.
We take reported issues seriously, and we investigate them promptly. Our internal reporting and investigations process promotes objectivity, confidentiality, dignity and respect. We respond to proven instances of misconduct with swift and appropriate disciplinary actions.
Global ethics and compliance oversight
Our chief ethics & compliance officer is responsible for corporate-wide activities, including those in the U.S. The chief ethics & compliance officer reports to the general counsel and periodically to the Audit Committee of our Board of Directors. The chief ethics & compliance officer manages a department that helps promote lawful behavior and supports the development and growth of a culture of integrity.
We implement modifications to our compliance program as appropriate. Our line managers provide ongoing compliance monitoring, auditing, and evaluation, and are expected to create an environment in which concerns are voiced, reported, and acted on.

Anti-bribery and corruption
We build relationships, we do not buy them. Our policies and practices prohibit bribery and corruption in all their forms.
Our employees are expected to follow the law to the letter and spirit. That means never promising, offering, paying, asking for or accepting anything of value to improperly influence decisions or actions with respect to our business. All employees complete anti-corruption training on an annual basis, and Board members complete anti-corruption training on a biannual basis.
We expect these standards from our third-party partners as well. We have a mature program led by the Office of Ethics and Compliance to conduct an assessment of suppliers’ practices regarding anti-bribery and corruption. The assessments are done on a recurring basis, and the program includes training for third parties on best practices that emphasize our commitment to acting with integrity and ethics. We strive to conduct business with individuals and organizations that share our commitment to high ethical standards and who operate in a socially and environmentally responsible manner. Our Business Partner Code of Conduct reinforces these standards.
Fair competition and open markets
We believe that our customers — and society — benefit from fair, free, and open markets. We compete aggressively but fairly and always within the bounds of responsible corporate behavior.
We expect our employees to promote customer choice, to foster positive business relationships, and to embody fair business practices. The strength of our products and services and the integrity of our practices distinguishes us from our peers.
Ethical marketing practices
We adhere to strict ethical sales and marketing practices. Our interactions with providers, customers and consumers are governed by laws, regulations, industry codes of conduct and our global Code of Conduct, which outlines our ethical expectations and commitment to integrity. We enforce these external and internal standards through our ethics and compliance program.
We strive to deliver accurate, balanced and useful information, including educational medical and scientific information, to healthcare professionals within the U.S. and globally.

Additionally, our Promotional Medical Forums are conducted by contracted external speakers. Speakers are selected based on defined, objective criteria that are directly related to the identified educational purpose of the Medical Forum and are aligned with applicable rules and regulations. Through contractual and compliance guardrails, the criteria for external speakers ensure that healthcare professionals provide substantive contributions while not interfering in their independent clinical decision-making. By attending one of our Organon Medical Forums, healthcare professionals participate in medical education on therapeutic and healthcare industry topics. The goal of this education is to provide substantive medical education to healthcare professionals with a bona fide educational need for information.
With our strict standards for conducting Organon Medical Forums, we comply with the AdvaMed Code of Ethics on Interactions with Healthcare Professionals, Pharmaceutical Research and Manufacturers of America (PhRMA) Code on Interactions with Healthcare Professionals, and FDA regulations, which ensure that product information is appropriately balanced to include the product’s potential benefits and risks and is consistent with approved product labeling. We believe that our marketing, sales and advertising activities make an important contribution to medicine by informing our customers of treatment options based on the most recent scientific information and findings from rigorous clinical studies and by supporting clinical skills that help ensure the safe and effective use of products. Our sales and marketing practices are governed by applicable laws and regulations, industry codes of conduct, our own global Code of Conduct, our corporate policies and procedures, and our ethics and compliance program.
Our policies require that our employees discuss our products honestly and accurately. All sales and marketing communications must be consistent with product labeling, with a fair disclosure of associated risks, and must help users understand and appropriately use our products. The communications should reflect the tone, content and meaning of the approved product labeling and refer only to approved products and the approved use of those products. They should also be appropriate based on the nature of the product, its use and the recipient of the information. Unsolicited requests for off-label information must be referred to employees who are specifically designated by the company to respond to these kinds of inquiries.
We are receiving an increasing number of requests for on-demand product information. In response, we offer resources and product information to healthcare providers on company websites and other digital platforms in certain countries.

In some countries, where permitted by law, we may directly inform patients and other consumers about diseases and available treatments that they may wish to discuss with their healthcare providers. We believe direct-to-consumer advertising contributes to greater awareness about conditions and diseases, which can benefit public health by increasing the number of patients who are appropriately diagnosed and treated.
Direct-to-consumer advertising
Where permitted, we believe that direct-to-consumer (DTC) advertising can be an important, helpful way to inform patients about diseases that may be relevant to them and about therapeutic options they may want to discuss with their physicians.
We try to help consumers achieve better health outcomes by delivering accurate, relevant and understandable information on disease prevention, identification, and potential treatment. We seek to adhere to the letter and spirit of FDA regulations and guidelines governing DTC promotion and follow a comprehensive set of internal policies and practices when engaging in DTC advertising within the U.S.
Under our DTC policies and practices, the information provided in our DTC advertising must:
- Contain appropriate product benefit and risk information.
- Be appropriately balanced and consistent with FDA regulations and use appropriate taste and tone.
- Be approved by our Promotion Review Committee, a governing body consisting of a cross-functional team of reviewers (generally including legal, medical affairs, and a regulatory representative from our Advertising Promotion Review Team) who help ensure that promotional material is clinically and scientifically accurate, compliant with applicable laws and regulations, and compliant with company policy.
We seek to adhere to updated 2019 PhRMA guidelines that require all DTC television advertising that identifies a medicine by name should include direction about sources, such as a company-developed website where patients can find information about the cost of the medicine, including the list price and average, estimated or typical patient out-of-pocket costs, or other context about the potential cost of the medicine. In addition, we include information on our U.S. Patient Assistance Program in all new U.S.-based DTC television advertisements for eligible products.
We inform and educate healthcare professionals about our products. We implement comprehensive programs to educate physicians and other prescribers about a new product for an appropriate period before starting product-specific DTC broadcast advertising in the U.S.
These principles and our practices generally reflect the PhRMA Guiding Principles on Direct-to-Consumer Advertisements about Prescription Medicines.
Fostering ethical practices
Our compliance program seeks to address and prevent inappropriate practices, and we evaluate our policies and practices as appropriate. Our practices are monitored, and compliance is enforced to help ensure that our interactions with customers and consumers help inform their decisions accurately and in a balanced manner. We believe that compliance with all policies governing scientific, business and promotion-related activities is a corporate and individual responsibility. Through our ethical behavior, we strive to ensure that scientific information predominates in prescribing decisions.
Our guiding principles for ethical business practices involving the medical and scientific community include the following:
- We provide current, accurate and balanced information about our products.
- We share sound scientific and educational information.
- We support medical research and education.
Our employees are prohibited from offering healthcare professionals, government officials and other third parties items of personal benefit, such as tickets to sporting events, support for office social events, or gift certificates for stores or golf outings. Where permitted under local laws and codes of practice, we may occasionally provide healthcare professionals with approved educational items that are not of substantial monetary value and that are intended primarily for educational purposes. Such materials may include medical textbooks, medical journals and anatomical models.
Our employees and others speaking on behalf of the company may give presentations specifically designed to provide the type of information that practicing healthcare professionals have indicated is needed and most useful in the treatment of their patients, in accordance with FDA regulations and the regulations of other countries in which the presentations or discussions are taking place. A company representative may offer occasional modest meals to healthcare professionals in connection with an informational presentation; however, such meals must be in accordance with local codes and regulations.
Compliance training and certification
As a condition of employment, all of our sales and marketing employees are required to be certified periodically on product information and sales and marketing practices.
In the U.S., employees who do not satisfactorily meet these training requirements may not conduct specific activities on their own and must repeat the training until they meet the requirements.
All new employees receive training and testing. And all our sales representatives must complete general sales and product training, even if they have advanced science or medical degrees. Training is specific to the country where an employee is based and covers the scope of the employee’s responsibilities in ensuring compliance with applicable laws and regulations.
Sales representatives are trained on anti-bribery and anti-corruption laws, such as the U.S. Foreign Corrupt Practices Act and the U.K. Bribery Act. Sales representatives in the U.S. are also required to understand, among other things, their responsibilities under the Anti-Kickback Statute, the U.S. Prescription Drug Marketing Act, the PhRMA Code on Interactions with Healthcare Professionals and all applicable FDA promotional regulations.
We evaluate and update the content for all marketing and sales training periodically to help ensure that it remains relevant and current.
Industry codes of conduct
Our sales representatives must provide truthful, non-misleading information in their interactions with the medical and scientific community.
Our compliance program is consistent with applicable laws and regulations and is aligned with the International Federation of Pharmaceutical Manufacturers and Associations (IFPMA) Code of Pharmaceutical Marketing Practices and with regional and country industry codes, such as the PhRMA Code, the AdvaMed Code of Ethics on Interactions with U.S. Healthcare Professionals and the Compliance Program Guidance for Pharmaceutical Manufacturers, published by the U.S. Department of Health and Human Services’ Office of Inspector General.
These codes were established by the broader pharmaceutical industry to address concerns raised by public officials and stakeholders in the healthcare community. Self-regulating industry codes of conduct, such as the IFPMA, European Federation of Pharmaceutical Industries and Associations, and PhRMA codes, set standards for the industry’s sales and marketing practices and help ensure that companies have adequate policies and procedures in place to comply with the codes.
We have incorporated the requirements of PhRMA’s Code on the Interactions with Healthcare Professionals into our already strong ethical business practices. For example, we follow the standards for commercial support of continuing medical education established by the Accreditation Council for Continuing Medical Education, and our compliance program requires that company representatives are periodically assessed to make sure they comply with relevant company policies and standards of conduct.
Learn more about our code of conduct and our business partner code of conduct.